Acid-Base Interpretation
An Efficient Method that Works Well for Hospital Rounds and the Boards
Proper acid-base interpretation is important to function well in a hospital setting. Throuhout your training, you have probably heard several approaches to acid-base interpretation. This is initially frustrating for such a standard topic, but the reason for this is that there are multiple ways to interpret acid-base abnormalities. The three main approaches to interpreting acid-base disorders. The first method is what we typically used for acid base interpretation and it is referred to as the Boston approach. The second type of method for interpreting acid base disorders is called the base excess, or Copenhagen approach. The last method for interpreting acid base disorders is called the Stuart approach and it is probably the most obscure out of all the methods.
What we will use for acid base interpretation is the Boston approach. Because it is the most widely used clinically. Specifically, the method we present works well for both clinical situations and for board exams and avoids cumbersome calculations.
Before we begin, there are a few terms we need to know. Acidemia refers only to a pH which is <7.35. Alkalemia only refers to a pH that is >7.45. Acidosis, on the other hand, is a physiological process which tends to lower the pH. For instance, a patient may have a metabolic acidosis which is producing acidemia. Alkalosis, in the same way, is any process that tends to raise the pH. The reason that this is important is that in mixed acid-base disorders, it is possible to have a combined metabolic acidosis and respiratory alkalosis which resulted in a pH of 7.28. In this example, yes a person does have an alkalotic disorder which is present in someone with acidemia, but only because the metabolic acidosis is overpowering the respiratory alkalosis. The terminology is important and will make you look smarter when talking to acid-base buffs like the pulmonologist.
Steps
The most useful first step to interpreting acid-base disorders is to calculate the anion gap first. A normal anion gap is 10mmol/L. You do not have to correct the anion gap for serum albumin. This was recommended in the past based on a 1998 paper that correlated the anion gap with unmeasured anions. The paper had flawed logic based on calculations and assumptions about albumin charge that only hold true in vitro. Contemporary studies show no added benefit of using an albumin-corrected anion gap to detect lactic acidosis. Therefore, the corrected anion gap is not needed.
If the anion gap is elevated, then the next step is to perform the Winters’ formula to calculate the expected PCO2 and progress to step number 5
If the anion gap is normal, then what you want to do is determine whether the patient has acidemia or alkalemia. Acidemia is defined by any pH less than 7.35. Alkalemia is defined by any pH greater than 7.45.
If the patient has acidemia, look at the bicarbonate level. If the bicarbonate level is low, then a metabolic acidosis is present. If the PCO2 is high, then respiratory acidosis is present. If the bicarbonate level is low and the pco2 level is high, then a mixed metabolic acidosis and respiratory acidosis is present.
If the patient has alkalemia, look at the bicarbonate level. If the serum bicarbonate level is high, then a metabolic alkalosis is present. If the PCO2 is low, then a respiratory alkalosis is present. If the serum bicarbonate level is high and the PCO2 level is low, then both a metabolic alkalosis and respiratory alkalosis are present.
The next thing you need to do is evaluate the patient for the level of compensation.
If the patient has a metabolic acidosis: use the winters equation which is: PCO2 = [1.5 x(HCO3-) +8] +/- 2. If a patient's PCO2 is higher than expected, then the patient has a concomitant respiratory acidosis. It's a patient's pco2 is lower than expected, then the patient has a concomitant respiratory alkalosis.
If the patient has a metabolic alkalosis: PCO2 increases 0.7 mmHg for each 1mmol/L increase in (HCO3-) +/-5. If a patient's PCO2 is higher than expected, then the patient has a concomitant respiratory acidosis. If a patient's PCO2 is lower than expected, then the patient has a concomitant respiratory alkalosis.
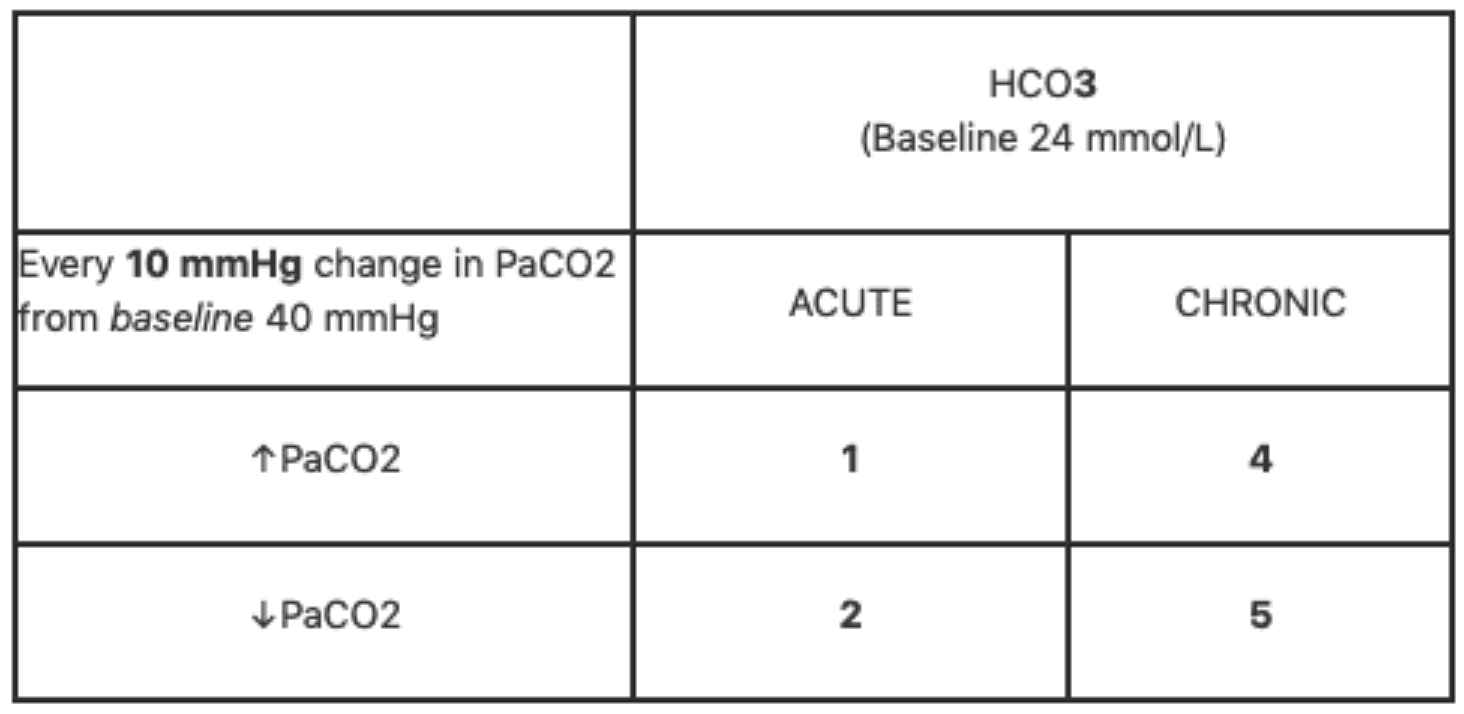
For respiratory disorders, use the following table to calculate the expected serum bicarbonate level for any change in PCO2. Acute respiratory acidosis will increase the bicarbonate level by 1mmol/L for every 10mmHg change in PCO2. Chronic respiratory acidosis will increase the bicarbonate level 4mmol/L for a 10mmHg increase in PCO2. Conversely, a decrease in PCO2 of 10mmHg will cause a decrease in serum bicarbonate of 2mmol/L and 5mmol/L for acute and chronic respiratory alkalosis, respectively. If the patient's bicarbonate level is lower than expected based on these rules, then the patient has a concomitant metabolic acidosis. If a patient's bicarbonate level is higher than expected, then the patient has a concomitant metabolic alkalosis.
If a patient has an anion gap metabolic acidosis, then the next thing to do is to calculate the Delta Gap. The Delta cap is essentially the absolute difference of the anion gap as compared to a normal anion gap of 10 divided by the absolute difference of the measured serum bicarbonate level as compared to a normal serum bicarbonate level of 24. The equation looks like this: [(AG-10)/(24 - measured bicarb)]. the rationale for this is that because 1 mmoL of lactic acid will require 1 mmoL bicarbonate for buffering, thus dropping the serum bicarbonate level in a 1:1 fashion. If the delta gap is <1, then it means that a NAGMA is also present. If the delta gap is >2, then a concominant metabolic alkalosis is present. in this last situation of a concominant metabolic alkalosis, it makes reasonable sense that if someone has an anion gap of 15, but a serum bicarbonate level of 23, then there must be some type of metabolic alkalosis present as well.
And so that is how acid-base interpretation is done.